28/05/21; C Store Decisions
While no industry or category has been spared the effects of the pandemic, the tobacco category has faced a special set of challenges. For one, the Food and Drug Administration’s (FDA) Premarket Tobacco Application (PMTA).
“The PMTA process has complicated the vapor world to another level beyond COVID that cigarettes didn’t see, smokeless didn’t see, cigars didn’t see,” said Circle K Tobacco Category Manager Kraig Knudsen, who oversees 387 of the Tempe, Ariz.-based chain’s stores.
On May 20, the FDA posted a list of deemed new tobacco products for which PMTAs were submitted by the Sept. 9, 2020, deadline. The list includes more than 6 million products, most of which are electronic nicotine delivery systems (ENDS).
Convenience retailers have long awaited guidance from the FDA, and this is a promising step forward for the industry and the e-cigarette subcategory.
“Vapor is still a very strong subcategory of tobacco in the OTP sector. But it’s been weakened by the FDA’s process,” Knudsen said. “I think once the dust settles and the whole PMTA thing is behind us, they will then be in a position to regain some share.”
In its announcement, the FDA noted the lists are not comprehensive, and retailers should still discuss with their suppliers the status of any tobacco product’s application or any product’s marketing authorization. For example, grandfathered products do not appear on the list, and tobacco products on the lists may be the subject of a warning letter.
There is a one-year period for the FDA to determine what products might remain on the market pending FDA review. If a negative action is taken on an application before Sept. 9, 2021, the product must be removed from the market or risk FDA enforcement. If a positive action is issued, the product will be listed on the positive marketing orders page and can continue to be sold, according to the terms specified in the order letter.
The agency’s Center for Tobacco Products (CTP) will hold a virtual meeting on June 11 with CTP Office of Science Director Matt Holman to improve public understanding of the policies and processes for tobacco product application review.
Moving Forward
Ultimately, regulation has stifled innovation in the category. “Even if you could come out with the best thing since sliced bread, you can’t even put it on the market until the FDA says you can,” Knudsen said.
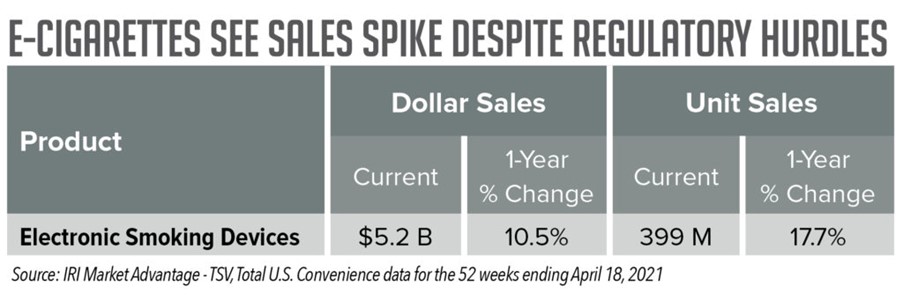
Still, according to IRI Total U.S. Convenience data, for the latest four weeks ending April 18, 2021, electronic smoking devices saw a 19.9% increase in dollar sales and a 24.5% increase in unit sales. And for the latest 52 weeks, the subcategory saw a 10.5% increase in dollar sales and a 17.7% increase in unit sales.

Rick Staley, merchandising manager for Nashville, Tenn.-based Tri Star Energy’s Twice Daily stores, noted its numbers are overall in line with IRI’s.
“At Twice Daily, customers are looking for alternative tobacco products,” he said. “We’ve noticed that nicotine pouches and vapor are both doing very well.”
And at ARKO Corp.’s GPM Investments, the vape subcategory is doing “extremely well,” said Kaitlyn Meara, GPM’s tobacco category manager, adding that customers are looking for variety in nicotine delivery, flavors and price points. The Richmond, Va.-based chain operates or supplies stores in 33 states and Washington, D.C., including both its 1,350 company-operated stores and approximately 1,600 dealer sites.
“Like in most other CPG categories,” Meara said, “we saw an increase in baskets due to customers making fewer trips.”
Now that people are starting to return to their routines and to more traditional work environments, much remains unknown about the way that consumers’ habits will shift moving forward.
Meara noted it’s extremely hard to predict the tobacco category as a whole, but she’s “excited about the innovation in the category with nicotine pouches and IQOS.”
“Modern oral, lozenges, gums, toothpicks, beverages — things with nicotine that you never dreamt up that are now options,” Circle K’s Knudsen said. “I think once we get past (the regulatory hurdles), there will be some new innovation. It’s exciting. … I can only guess where the category would be if it had not hit this roadblock.”
Subscribe to our free mailing list and always be the first to receive the latest news and updates.
